Growing Body of Clinical Evidence Demonstrating Aurix’s Efficacy
Nuo Therapeutics, Inc. (NUOT – $0.26) earlier this week presented new clinical evidence that supports its leading-edge therapies for wound care. The Company’s star product, Aurix is the first platelet and plasma therapy system that’s cleared by the FDA for the management of ulcers and wounds of all types (diabetic foot ulcer, pressure ulcer, venous leg ulcer, etc.) and all severities.
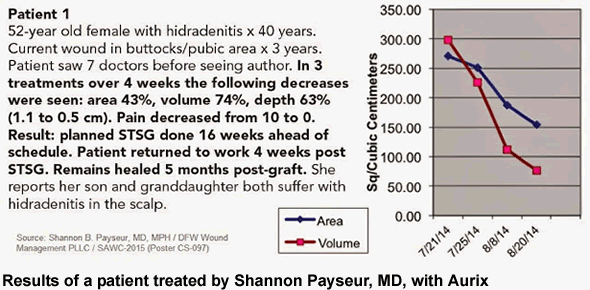
At the 28th Annual Symposium on Advanced Wound Care Spring Meeting, held in San Antonio, Texas, Shannon Payseur, MD, who specializes in wound care at DFW Wound Management PLLC, highlighted case studies for three patients with chronic infected wounds caused by chronic diseases.
During treatment, Aurix was applied to each patient’s wound once it demonstrated response to management of infection. Each patient received three treatments of Aurix, and in each case, experienced significant decreases in the area, volume and depth of their respective wounds, decreasing the anticipated time to resolution of their wound. Importantly, in the two hidradenitis cases, patients reported a reduction in pain from 10 to 0 and 10 to 1, respectively.
Phase 4 Study
To further demonstrate the effectiveness of Aurix, the Company recently initiated a new clinical study that is comprised of three randomized controlled protocols. In the three open-label, randomized studies, diabetic foot ulcers, venous leg ulcers and pressure ulcers, respectively, will be treated using Aurix and standard care, and compared one-to-one with patients receiving Usual and Customary Care.
The Centers for Medicare and Medicaid Services (CMS) has approved these amended protocols under its Coverage with Evidence Development (CED) Program. Under this program, CMS will reimburse for Aurix when health care providers agree to collect their Medicare patients’ treatment data for inclusion in the study database.
“We are pleased to have now refined and finalized with CMS the three protocols we will use to populate what will be one of the largest wound care studies of its kind in the U.S.,” said Martin Rosendale, CEO of Nuo Therapeutics.
Kickstarting Sales
As we mentioned in our initial recommendation for Nuo Therapeutics (then called Cytomedix), sales for Aurix were initiated in October 2014 by appointing two experienced sales people from Shire Plc. Dean Tozer brings more than 25 years of experience in the global healthcare industry and was responsible for the acquisition and reintroduction of Dermagraft, one of the best-selling wound-care products into the U.S. market. And Jennifer Linsky was a National Accounts Manager and National Manager of Market and Business Development for Shire Regenerative Medicine.
Because the team was only appointed in the fourth quarter of 2014, there wasn’t enough time to have an impact on last year’s sales. As a result, total revenues for the year were $7.8 million, compared to $11.6 million in 2013.
Total operating expenses for the year were $24.4 million, compared to $21.2 million in 2013. The increase in operating expenses was attributable to the expansion of Nuo’s commercial, marketing, and clinical organization. The Company reported a net loss for the year of $18.9 million, or $(0.16) per share, compared with $20.2 million or $(0.20) per share a year ago.
Cash and cash equivalents as of December 31, 2014 totaled approximately $15.9 million, compared with approximately $3.3 million as of December 31, 2013.
Dean Tozer, Chief Commercial Officer of Nuo Therapeutics, said: “In the five months since we launched the newly-branded Aurix at the Symposium on Advanced Wound Care (SAWC) Fall Meeting, our commercial team has been aggressively pursuing a variety of sales opportunities with customer targets, focusing on those responsible for making purchasing decisions at Veterans Administration hospitals and outpatient wound care centers.
“Thus far in 2015, we have continued to steadily advance the Aurix commercial program through CMS approval of our amended CED protocols and initiation of our Au Study. These recent milestones give us confidence in our ability to take advantage of the large chronic wound care market opportunity, and we anticipate that the substantial efforts we have put forth have the potential to generate meaningful returns over time.”, added Mr. Rosendale.
Conclusion
There’s a growing body of clinical evidence demonstrating Aurix’s efficacy as a viable treatment option for the treatment of ulcers and wounds of all types and severities. Customer response to Aurix in treating these very complex wounds has been overwhelmingly positive.
With more than 6 million wounds that need treatment every year, the wound care market in the United States is huge.
Nuo Therapeutics has the only FDA approved product for all types of wounds and all severities. In addition, it has a top sales team with lots of experience in the wound care field. We expect Aurix sales to pick up in the course of this year. Hold on to your shares.
| Sign up for our weekly e-mail newsletter and be the first to receive our best ideas and updates. |
| For important disclosures, please read our disclaimer. |
