Cytomedix All Set To Capture U.S. Wound Care Market
Warning: this article contains images that some might find distressing.
I’ll start this recommendation with a confession. I only like biotech and medical device companies after they’ve received regulatory approval. The trial process before a product or drug can be brought to the market, is just too time and money consuming for my taste. Even more importantly, despite fine test results a regulatory body, like the FDA, can always reject a drug or ask for more trials. I learned the hard way…
Several years ago, I invested in a couple of biotechs with little success. And because being a good investor includes learning from past mistakes, I’ve steered away from those types of stocks since.
Instead, I search for companies that already have obtained regulatory approval, but for some reason haven’t reached their potential yet. In January of this year, we featured IsoRay Inc (ISR – $1.59) on Smallcaps.us, a medical technology company and innovator in seed brachytherapy and medical radioisotope applications. Although the company had received FDA clearance to treat certain brain cancers in a revolutionary way, sales hadn’t picked up yet.
We sensed an opportunity and recommended to purchase shares at $0.75. Less than two months later, IsoRay was trading at $3.30, up 340% from our recommended price.
A Similar Company
We’ve found another biomedical company, which is currently in a somewhat similar situation as IsoRay was back in January. It owns a revolutionary product that’s cleared by the FDA, but has failed to generate meaningful sales so far.
The Aurix (up until a few weeks branded as AutoloGel) wound care system from Cytomedix, Inc. (CMXI – 0.36) is the first platelet and plasma therapy system that’s cleared by the FDA for the management of ulcers and wounds of all types (diabetic foot ulcer, pressure ulcer, venous leg ulcer, etc.) and all severities.
The Aurix System utilizes a proprietary and unique technology that enables the rapid isolation and activation of platelet rich plasma (PRP) from a patient’s own blood. The PRP is subsequently processed to produce a bioactive gel for application to the wound bed. In normal healing, platelets migrate from the blood into the wound site where they serve as the primary source of growth factors for effective wound healing. In chronic wounds, blood supply may be impaired and the delivery of platelets is impeded, disallowing adequate concentrations of growth factors.
Aurix produces visible results in days, not weeks and is effective at all stages of wound care for non-healing wounds.
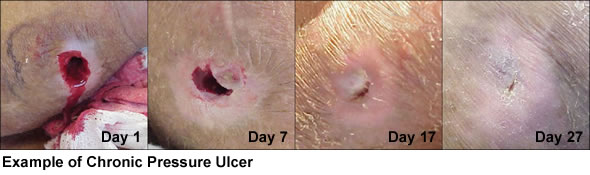
Cytomedix’s clinical studies have shown that Aurix rapidly and more effectively improved healing compared with control-treated wounds. This has been demonstrated in a variety of clinical studies including a systematic review of 21 comparison studies and a number of other observational and case series publications.
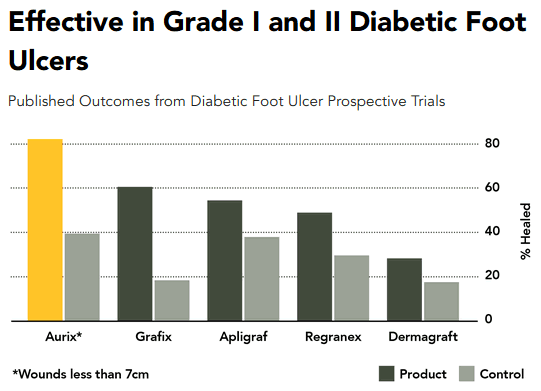
Transformation
The study results indicate that Aurix is the top product in the multi-billion dollar U.S. chronic wound care market. All Cytomedix needs is an outstanding sales organization to spread the word on its product.
That’s exactly what the company has been up to in 2014. Over the last 10 months, Cytomedix has transformed its organizational focus, with an emphasis on developing the commercial capabilities necessary to support the nationwide launch of Aurix. The company completed a strategic realignment by out-licensing non-core assets, closing its R&D facility and raising $37 million to build and equip a world-class commercial team.
Cytomedix hired two experienced sales people from Shire Plc. to lead this team. Dean Tozer brings more than 25 years of experience in the global healthcare industry and has an established track record of successfully launching and marketing wound care products. He was responsible for the acquisition and reintroduction of Dermagraft, one of the best-selling wound-care products into the U.S. market. Jennifer Linsky also joins Cytomedix from Shire Regenerative Medicine, where she was a National Accounts Manager and National Manager of Market and Business Development.
Conclusion
The timing of this recommendation is ideal as Cytomedix recently announced that it’s ready to begin full scale product promotion with a direct sales team of nearly twenty representatives. The company intends to drive demand within both hospital outpatient wound care centers and Veterans Affairs facilities.
The wound care market in the United States alone is huge, with more than 6 million wounds that need treatment every year. The broad Aurix label provides clearance from the FDA for the product to treat multiple types of chronic wounds.
As of June 30, 2014, the company had a comfortable cash and cash equivalents position of approximately $25.0 million.
Under the CED (Coverage with Evidence Development) program, Medicare reimburses for promising new technologies while further evidence is developed. As such, Aurix is nationally covered for the approved protocols under the CED program.
Cytomedix has 124 million shares outstanding and has a market cap of about $44 million. Its shares are currently trading near the stock’s 52-week low, so there’s lots of upside potential. Buy recommendation.
Note that the company intends to change its corporate name to Nuo Therapeutics, Inc., following shareholder approval of the name change proposal at its Annual General Meeting of the Company’s shareholders on November 12, 2014.
| Sign up for our weekly e-mail newsletter and be the first to receive our best ideas and updates. |
| For important disclosures, please read our disclaimer. |
